BioAge labs is one of the most interesting longevity biotech startups, with a new drug that looks to prevent muscle aging (and muscle degradation during bedrest, fat loss, etc.).
See: BioAge Results for Phase 1b Clinical Trial on Anti Muscle-Aging Drug
And while the drug candidate is currently in clinical trials - I’m surprised to see the company filing for an IPO.
An IPO before any revenue… reminds me of the good 'ol Dot Com era of public markets ![]() Take the money when you can.
Take the money when you can.
I guess they are repositioning the company a bit from “longevity” towards more of an “obesity focus” (since their drug may protect against muscle loss that can happen with people taking GLP1 inhibitors and losing large amounts of weight), which will play well in the capital markets given the success of the GLP1 drugs.
I just hope that this is truly more just of a “repositioning” of the company into the obesity market, and not a “pivot” away from longevity. This IPO could be a good thing for the longevity market generally, in that it represents an increased flow of funding into the market, and increased funding specifically for the longevity-oriented drugs that BioAge labs has discussed in the past. The additional funds should help the company bring its products to market faster, so this should be a good thing for longevity enthusiasts.
BioAge Labs, a Phase 2 biotech developing obesity therapies by targeting metabolic aging, announced terms for its IPO on Wednesday.
The Richmond, CA-based company plans to raise $135 million by offering 7.5 million shares at a price range of $17 to $19. Existing investor Sofinnova Venture Partners plans to invest $15 million in a concurrent private placement. At the midpoint of the proposed range, BioAge Labs would command a market cap of $602 million.
The company’s leading candidate, azelaprag, is an orally available small molecule that has shown encouraging results in preclinical and early clinical trials, particularly in enhancing weight loss when combined with GLP-1R agonists. Beyond weight loss, azelaprag may also improve body composition and muscle function by leveraging a unique technology platform and proprietary human datasets to identify promising targets linked to molecular changes associated with aging. BioAge Labs is currently conducting Phase 2 clinical trials to evaluate azelaprag’s potential when combined with existing obesity treatments, aiming to develop an all-oral combination therapy for obesity. Topline data from the first of these trials is expected in the 3Q25. It is also advancing brain-penetrant NLRP3 inhibitors for neuroinflammation-driven diseases, with plans to submit an IND in the 2H25.
BioAge seeks up to $587 mln valuation in US IPO
Full S-1 IPO Registration document below.
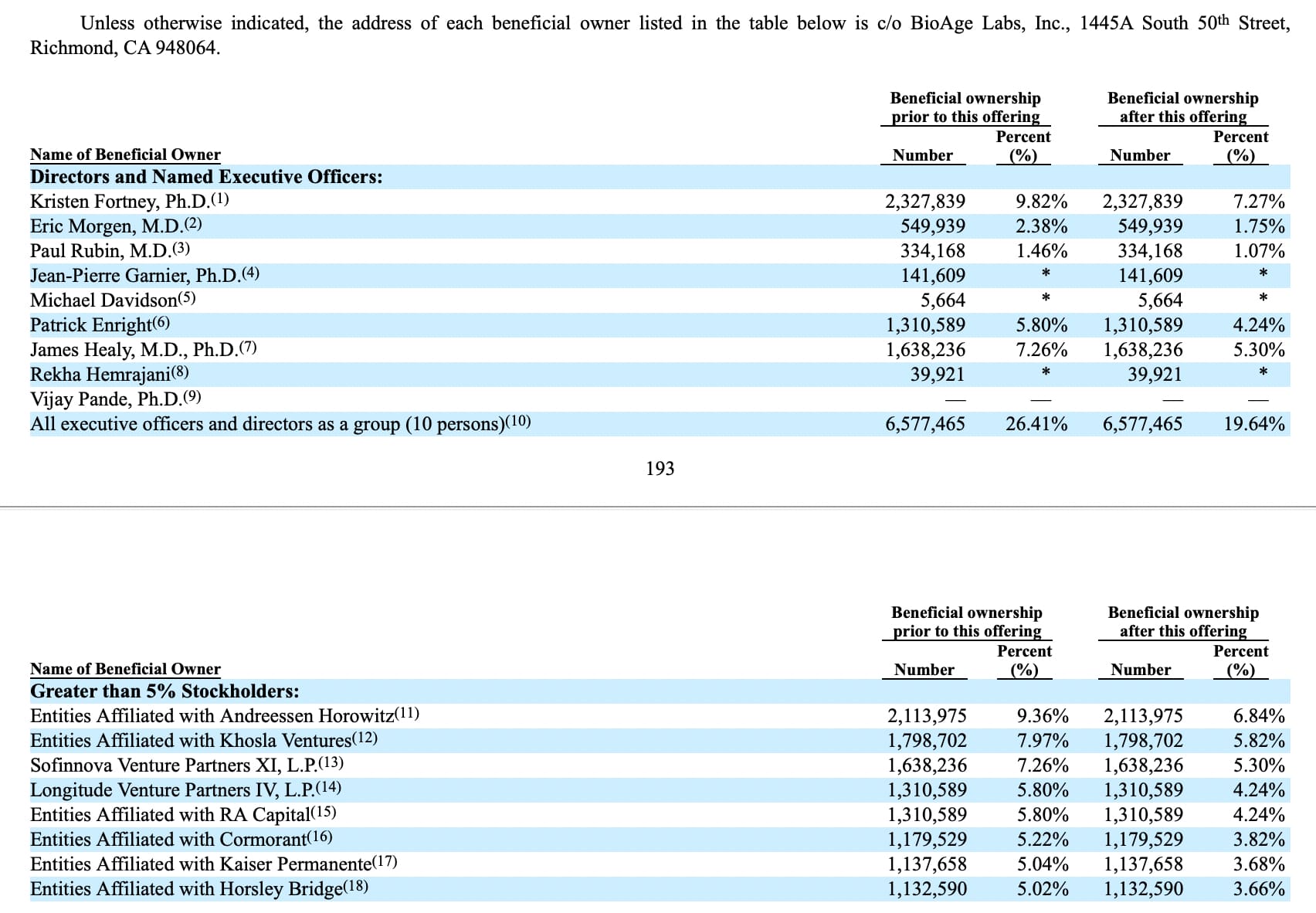
This should motivate many more people to jump into on the longevity biotech startup world. The founder of BioAge Labs, Kristin Fortney (out of University of Toronto, and Stanford U.) will hold about 7% of the company after the IPO, with a value of about $ 40 Million+.
Overview
We are a clinical-stage biopharmaceutical company developing therapeutic product candidates for metabolic diseases, such as obesity, by targeting the biology of human aging. Our technology platform and differentiated human datasets enable us to identify promising targets based on insights into molecular changes that drive aging. Our primary focus is metabolic disease, one of the greatest global healthcare challenges. Azelaprag, our lead product candidate, is an orally available small molecule that has been well-tolerated in 265 individuals across eight Phase 1 clinical trials. In preclinical obesity models, azelaprag demonstrated the ability to more than double the weight loss induced by a glucagon-like-peptide-1 receptor (GLP-1R) agonist while also restoring healthy body composition and improving muscle function. These preclinical results are supported by our Phase 1b clinical trial in older adults on bed rest where we observed decreased muscle atrophy, preservation of muscle quality and improved metabolism in subjects treated with azelaprag over a 10-day period. We plan to assess azelaprag’s potential to drive significant improvements in weight loss when combined with a GLP-1R agonist in two Phase 2 clinical trials. While the results of these preclinical studies and early clinical trials have demonstrated the potential use of azelaprag for the treatment of metabolic disease, they may not be predictive of the results of later-stage clinical trials. The ongoing STRIDES clinical trial will assess azelaprag in combination with tirzepatide, marketed as Zepbound® by Eli Lilly and Company (Lilly), with topline results anticipated in the third quarter of 2025. The second Phase 2 clinical trial will assess azelaprag in combination with semaglutide, marketed as Wegovy® by Novo Nordisk, with initiation expected in the first half of 2025 and topline results expected in the second half of 2026. We believe these trials will directly support our ultimate therapeutic goal of developing an all-oral combination product for obesity. We also intend to initiate an insulin sensitivity proof-of-concept trial of azelaprag monotherapy in the first half of 2025 to support potential indication expansion. We expect to report topline results from this proof-of-concept trial in the second half of 2025. We are also developing orally available small molecule brain-penetrant NLRP3 inhibitors for the treatment of diseases driven by neuroinflammation. We anticipate submitting an Investigational New Drug application (IND) for an NLRP3 inhibitor in the second half of 2025 and, if cleared, initiating a Phase 1 clinical trial in the first half of 2026.
https://www.sec.gov/Archives/edgar/data/1709941/000119312524221367/d835745ds1a.htm