George sutphin has a paper (he used to be in Kaeberlein lab
On the benefits of the tryptophan metabolite 3-hydroxyanthranilic acid in Caenorhabditis elegans and mouse aging
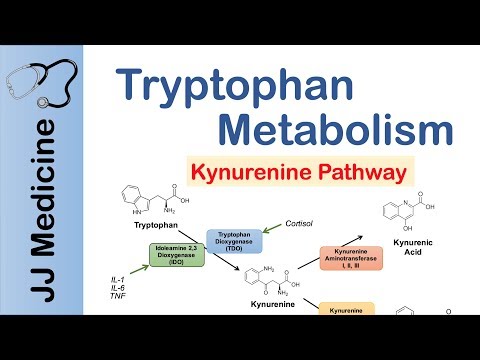
Tryptophan metabolism through the kynurenine pathway influences molecular processes critical to healthy aging including immune signaling, redox homeostasis, and energy production. Aberrant kynurenine metabolism occurs during normal aging and is implicated in many age-associated pathologies including chronic inflammation, atherosclerosis, neurodegeneration, and cancer. We and others previously identified three kynurenine pathway genes—tdo-2, kynu-1, and acsd-1—for which decreasing expression extends lifespan in invertebrates. Here we report that knockdown of haao-1, a fourth gene encoding the enzyme 3-hydroxyanthranilic acid (3HAA) dioxygenase (HAAO), extends lifespan by ~30% and delays age-associated health decline in Caenorhabditis elegans. Lifespan extension is mediated by increased physiological levels of the HAAO substrate 3HAA. 3HAA increases oxidative stress resistance and activates the Nrf2/SKN-1 oxidative stress response. In pilot studies, female Haaoknockout mice or aging wild type male mice fed 3HAA supplemented diet were also long-lived. HAAO and 3HAA represent potential therapeutic targets for aging and age-associated disease.
Open access paper:
https://www.nature.com/articles/s41467-023-43527-1
====
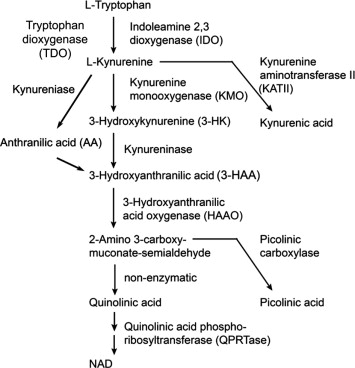
note quinolinic acid is neurotoxic…
Our data demonstrate that 3HAA is protective against ROS in the context of aging C. elegans. 3HAA has a complex history with oxidative stress, with earlier studies linking 3HAA to ROS generation and/or oxidative damage28,29,30,31 and more recent studies reporting antioxidant properties for 3HAA32,33,34,35,36,37,38,39. These studies vary widely in the oxidant properties examined, presence of added metals, cell type, and cellular context. 3HAA can auto-oxidize under specific conditions, including the presence of Cu2+, Fe3+ or alkaline pH28. Each of these variables is likely critical to understanding redox properties of 3HAA in the context of aging, and the particular importance of copper and pH were recently confirmed in silico15. Our data that 3HAA can both directly degrade H2O2 and promote endogenous H2O2 production suggest that both sides of this debate are likely correct in context. Given both that 3HAA is protective against oxidative stress and the complex interaction between haao-1 and genes in other aging pathways (Fig. 1g), we speculate that 3HAA may also impart protection against other forms of cellular stress.