Some experts have said that we may also look back on GLP-1 drugs as one of the greatest modern advancements in the treatment of chronic conditions. They’ve already shown promise for cardiovascular, liver and kidney disease, and some are now being tested on Alzheimer’s, sleep apnea, PCOS—even addictive behaviours, like gambling. That is a huge scope.
Are these drugs going to help all of these different conditions in a meaningful way? That’s unlikely. We don’t yet have enough data to say we should put this stuff in the drinking water, but there are encouraging signs of their benefits. Right now, we have excellent data for Type 2 diabetes, heart disease and obesity. We’re gonna get some excellent data for kidney disease in the next few months, then heart failure in the summer. We’ll have to wait a year or two for Parkinson’s and Alzheimer’s.
Phase 3 trial of exenatide (the first approved GLP1RA) for Parkinson’s ended last month. So we’ll get data way sooner for PD.
I lost 65 lbs of scale weight and gained 1kg of lean mass while on tirzepatide (Mounjaro). I got DEXA scans. If one gets adequate protein and lifts heavy weights at least 2x a week (I was doing 3-4, progressive overload), one does not have to lose muscle. There are many similar anecdotes online. Judging by the online forum participants, most people on these drugs are obese women who don’t lift weights. Of course they lose muscle. These are the people who are letting the drug do all the work for them (not even tracking calories and macros, etc.)
Btw, tirzepatide is generally regarded by those who have tried both to be vastly superior to semaglutide in terms of effectiveness and the side effect profile. Retatrutide is even better in some respects because one tends to have more energy (less fatigue) on it. Tirz usually begins working within a couple days of the initial injection, but with sema you have to slowly increase the dose because the side effects are otherwise often intolerable. Reta usually takes 2-4 weeks to kick in, but when it does, you simply have zero appetite. I wouldn’t waste time with semaglutide.
fatigue while on it?
I think the fatigue comes from allowing caloric intake to drop too low. It’s easy to not eat enough and especially not eat enough carbs when you’re trying to prioritize hitting a protein target, because after protein and fat, you might not have a lot of room left in your energy budget for carbs. I suppose the keto people wouldn’t have this issue, but I don’t function well on a keto diet and needed some carbs.
I tracked calories in Cronometer, weighing and measuring everything, and tried to not let my deficit get too low.
The other thing was to exercise daily no matter what (well, sometimes Sunday off). I mostly just alternated cardio with weights, and I didn’t slack off in terms of intensity with either.
Now with retatrutide things are a little different because the glucagon receptor agonist activity seems to provide an energy boost (a common anecdote and my experience). Retatrutide feels better than tirzepatide, I think, partially because of this. You can eat more on reta but the pounds just fall off as if they’re getting torched in a metabolic furnace.
I’ve been taking oral semaglutide (Rybelsus) for the past month, with mixed results (I know it’s not nearly as bioavailable or effective as the injectable semaglutide, much less tirzepatide or retatrutide, but at least it’s branded Rybelsus (not a compounded generic), it’s affordable, and I know what I’m getting.
Anyway, at both the 7mg dose and 14mg, I’m getting significant sleep disruption per both subjective experience and per my Oura Ring Gen 3, which shows decreased sleep duration/quality, increased heart rate (about 10 BPM) and significantly decreased heart rate variability (HRV). The 7mg dose is more tolerable but also less effective suppressing my appetite. I increased to the 14 mg dose for a week, and the appetite suppression was far better, but the sleep disruption/fatigue was too much to handle, so I’ve gone back down to 7mg for the time being.
I’ve lost 10 pounds, all of which should be fat given that I’m still doing near-daily resistance training and maintaining protein intake via whey isolate powder, but I think I’ve plateaued with the weight loss on the 7mg dose. I’m going to try a few things to see if I can get HRV and heart rate back under control as well as daytime fatigue (which for me seems much more related to the sleep disruption than caloric deficit since I feel markedly better after a mid-day nap, but I’d prefer not to have to take naps). If I can make a dent in those parameters, I’ll try the 14mg dose again and see how it goes.
semaglutide in addition to dapagliflozin 10 mg to decrease further my HOMA-IR (from 1.4 to below 1).
Do you know how much the insulin may go up? If Homa-Ir is the goal it might negate some of the improvement in glucose optimization.
—
As discussed elsewhere insulin up might also be anti longevity - so might want to measure that extra if you do trial it
—
Btw, what do you glucose patterns look like, have you done period with a CGM?
—
Are there any reasons you don’t consider acarbose to be an option?
Do you know how much the insulin may go up? If Homa-Ir is the goal it might negate some of the improvement in glucose optimization.
No idea. That’s why I wanted to test one month or two then measure insulin (and therefore HOMA-IR).
Btw, what do you glucose patterns look like, have you done period with a CGM?
Yes, I’ve used many CGMs over the past 12 months. I could share screenshots but I think it’s useless because you need to know what I ate on that day to understand whether a peak/pattern is “normal” or not.
Are there any reasons you don’t consider acarbose to be an option?
I take 50 mg acarbose whenever I have a more carb heavy meal (mostly when dining out). I tried to take it 2x daily but it didn’t improve things that much.
daytime fatigue (which for me seems much more related to the sleep disruption than caloric deficit
That’s a good point, and tirz and reta also tend to cause a heart rate increase (especially reta) and sleep disruption at least in the beginning. This is very commonly reported on the forums. And I also had a drop in HRV.
I only experienced the sleep issues from tirz for about 2 weeks before adjusting to it, but the first few days were really bad. I wonder if you would adjust to the 14 after a while.
The nice thing about injections is that they’re easy to adjust, of course. The jump from 2.5mg/wk to 5mg a week of tirz was brutal given that it was a doubling, so I just reduced it down to 3.5 for a few weeks. I also didn’t stick to once per week, adjusting that as hunger warranted. I suppose one can play with frequency with pills or injections though.
More generally, it’s worth mentioning that these medications certainly make weight loss possible, but I don’t think they make it as easy as people may think because the side effects really can be difficult to ensure for months on end. I was somewhat nauseous most days, but usually just a little bit. Enough to be uncomfortable but not incapacitated. It wasn’t fun and if I had to do it over again, I think I would have tried taking breaks.
I’ve used many CGMs over the past 12 months. I could share screenshots but I think it’s useless because you need to know what I ate on that day to understand whether a peak/pattern is “normal” or not.
I think it is valuable to help provide a picture, could you perhaps comment on the general picture over weeks and months re eg:
- what’s is the glucose level generally like the hour before you wake up?
- how high are your peaks after (a) normal, non-big carb meals and (b) after big carb meals?
- how many times per week do you go above 130? Above 140? Above 150?
- what’s the standard deviation?
- what’s the average glucose level from the CGM? (How does that compare to and what is your HbA1c?)
Thanks for asking good questions ![]()
- what’s the standard deviation?
- what’s the average glucose level from the CGM? (How does that compare to and what is your HbA1c?)
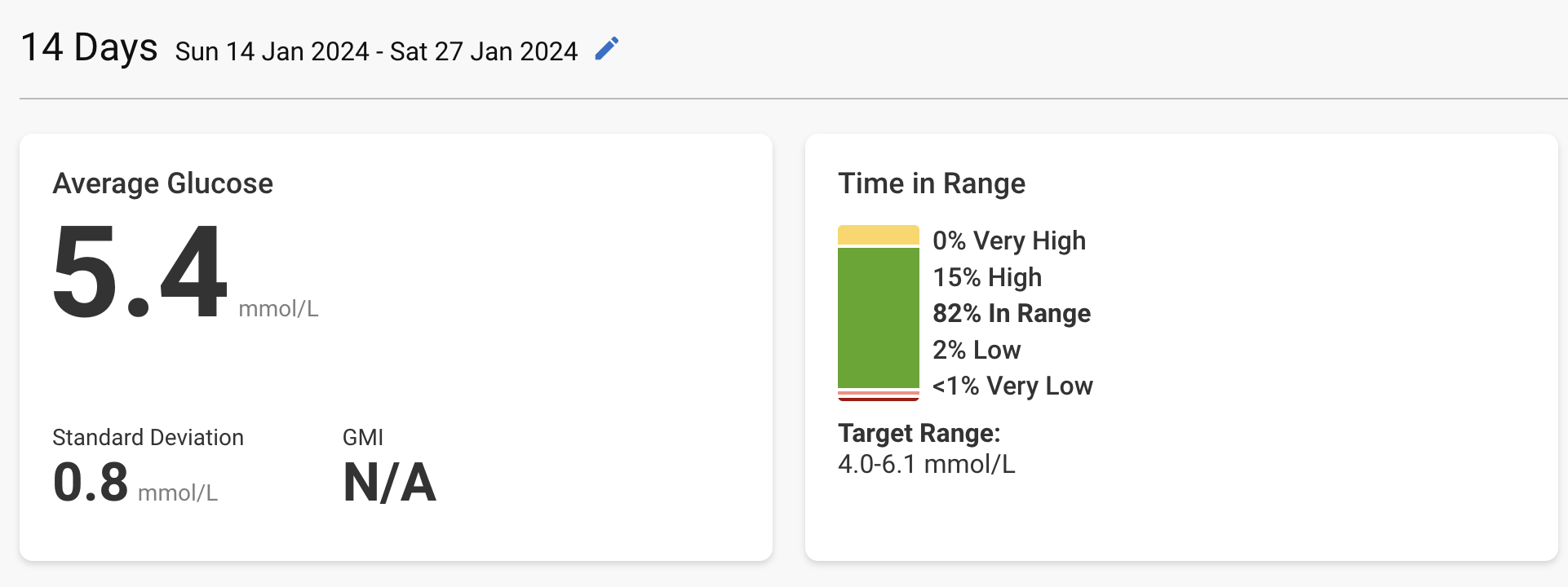
My most recent CGM data (from Dexcom G7, the most accurate device with the FreeStyle Libre 3). 4.0–6.1 mmol/L = 72–110 mg/dL:
The average given by the CGM (5.4 mmol/L = 97 mg/dL) is equivalent to Hb A1c of 5.0% (source). My latest Hb A1c from early March was 5.1%. So, CGM and blood test are in agreement.
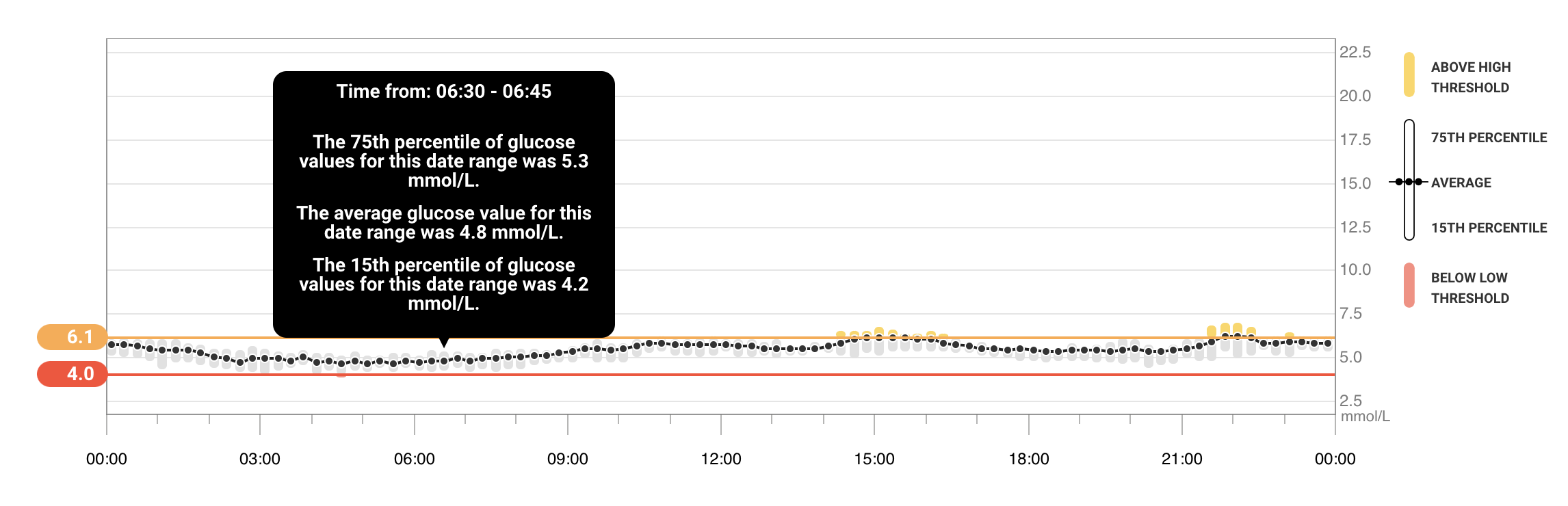
- what’s is the glucose level generally like the hour before you wake up?
Per CGM: 4.8 mmol/L = 86 mg/dL
My fasting glucose (blood test) from early March was 4.6 mmol/L. Here again, CGM is accurate.
- how high are your peaks after (a) normal, non-big carb meals and (b) after big carb meals?
Before starting drugs (dapagliflozin and acarbose), peaks as high as 10 mmol/L (= 180 mg/dL), sustained for a few hours:
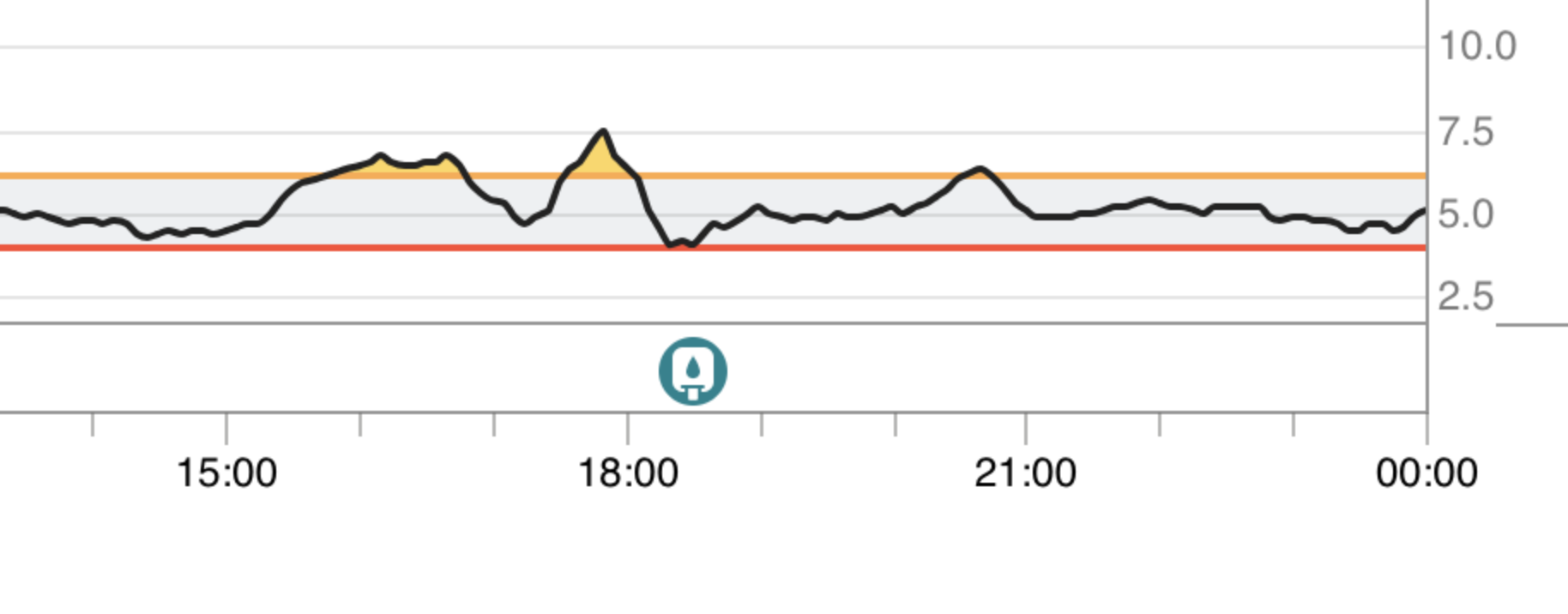
With acarbose 50 mg (but no dapagliflozin), big carb late lunch starting at ~14:30pm, peak of 7.5 mmol/L = 135 mg/dL
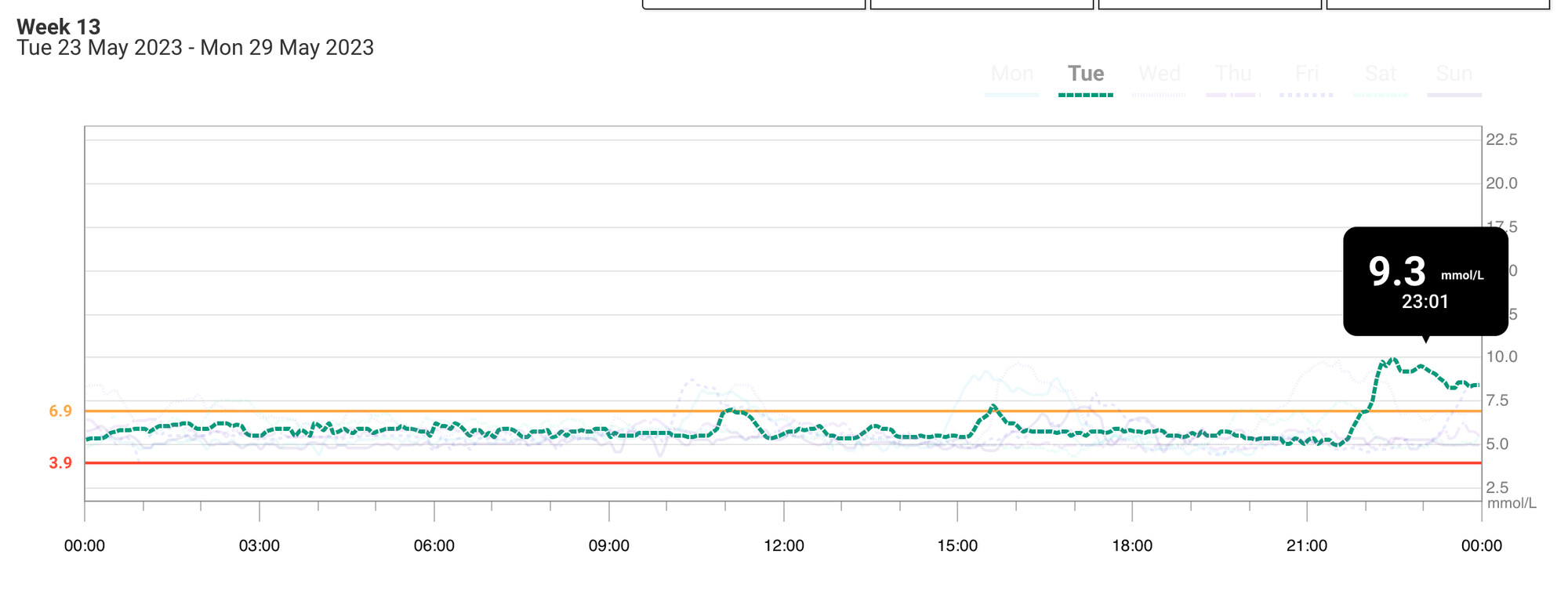
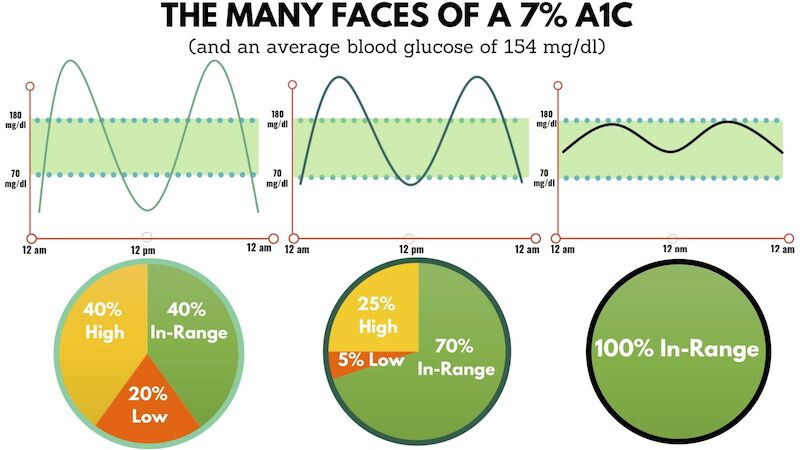
The peak is OK. But I’m “yo-yo-ing” a lot, and when this happens, I feel terrible. That’s why Hb A1c isn’t a great KPI; the same average can correspond to different patterns:
When it’s “yo-yo-ing” it can drop quite quickly, leading to CGM alerts and hypoglycemia:
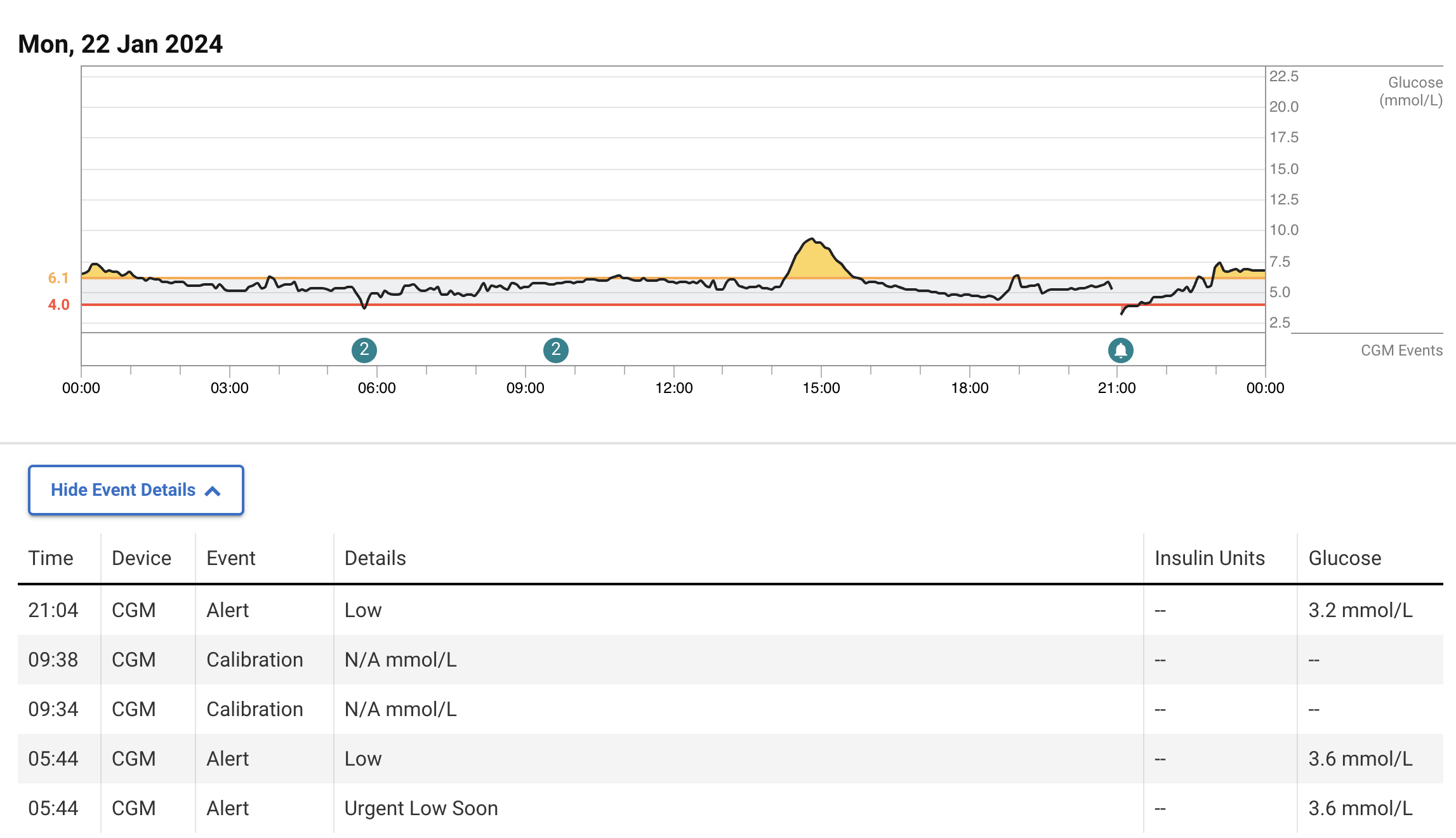
Dapagliflozin has significantly reduced the frequency of these hyperglycemic and hypoglycemic episodes but they still happen (also at night):
- how many times per week do you go above 130? Above 140? Above 150?
Using the last 7 days of my latest CGM data, with dapagliflozin 10 mg daily + acarbose 50 mg just before “big” meals + “good carb” diet (no white rice but whole grain quinoa OK for instance):
- ≥ 130 mg/dL (7.2 mmol/L): 5 times/week
- ≥ 140 mg/dL (7.8 mmol/L): 3 times/week
- ≥ 150 mg/dL (8.3 mmol/L): 1 time/week
Latest HOMA-IR from early March: 1.4 (glucose: 4.6 mmol/L & insulin: 39.8 pmol/L).
It’s counter-intuitive, but GLP-1RAs seem to be able to reduce post-meal hypoglycemic episodes and improve glycemic variability as they prevent the excessive peak of endogenous insulin (see The efficacy of GLP-1RAs for the management of postprandial hypoglycemia following bariatric surgery: a systematic review). Also: “Emerging evidence indicates that semaglutide could have disease-modifying actions on insulin resistance (HOMA-IR) and the early stage of prediabetes and T2D” (Real world effectiveness of subcutaneous semaglutide in type 2 diabetes: A retrospective, cohort study (Sema-MiDiab01) 2022). So even though GLP-1RAs might increase insulin, if they significantly reduce HOMA-IR it means that they don’t increase it that much (as HOMA-IR = glucose x insulin / constant). Anyway, it’s worth trying.
Very cool summary.
High level it seems like you have definitely improved your glucose dynamics a lot.
(Depending how how you sleep/where you were the CGM the nigh time drops might just be about sleeping on the arm where you are wearing the CGM).
Based on the data you shared I’d probably be more focus on the insulin part of the equation than the glucose. You might still benefit from some more glucose optimization, but ideally not if the cost comes at increased insulin.
Wish we had continuous insulin measurements… I could see how someone having big problems with glucose (more than you have on the SGLT2i + Acar) might lower their insulin peaks via knocking down the glucose more when on GLP1-RAs. Might be the case for you too, but would def try and get as many insulin datapoints before and after the GLP1-RA experiment if you decide to do it.
Would also try and see if there is data in non-obese/non-bariatric surgery/non-diabetes humans and mice about the direction of insulin on GLP-1 meds.
Btw, what are your IGF-1 levels? If they are not as low as you’d like from a longevity phenotype you might be want to be a bit more concerned about risking to increase your insulin levels.
(the issue I don’t think we know enough about is also to what extent the GLP-1 impacted insulin levels are increased 24/7 and not just associated with the postprandial pattern of going up, but then down again).
Lastly, have you explored doing some of the non-medical things to lower glucose (more fiber, order of eating different foods, some movement after meals, using vinegar and things like cinnamon going into meals, finding ways to destress / lower cortisol levels, etc, etc, etc)?
(Depending how how you sleep/where you were the CGM the nigh time drops might just be about sleeping on the arm where you are wearing the CGM).
I didn’t know that it could cause hypos. Do you have sources on this by any chance?
Based on the data you shared I’d probably be more focus on the insulin part of the equation than the glucose. You might still benefit from some more glucose optimization, but ideally not if the cost comes at increased insulin.
Besides the longevity risk–benefit assessment, I want to stop my post-(big) meal symptoms and improve my quality of life. If the cost is increased insulin, I might be OK with it.
Would also try and see if there is data in non-obese/non-bariatric surgery/non-diabetes humans and mice about the direction of insulin on GLP-1 meds.
Yes, I couldn’t find such data. The results of semaglutide for CKD will be published soon, so hopefully, the full results should show insulin levels before and after among non-obese and non-diabetic people.
Btw, what are your IGF-1 levels? If they are not as low as you’d like from a longevity phenotype you might be want to be a bit more concerned about risking to increase your insulin levels.
I think I’ve never measured it. What should I read about this?
Lastly, have you explored doing some of the non-medical things to lower glucose (more fiber, order of eating different foods, some movement after meals, using vinegar and things like cinnamon going into meals, finding ways to destress / lower cortisol levels, etc, etc, etc)?
I do ~ 80% of these things (I’m also a Zoe client). It helps, but not enough.
Forgot to answer this:
(the issue I don’t think we know enough about is also to what extent the GLP-1 impacted insulin levels are increased 24/7 and not just associated with the postprandial pattern of going up, but then down again).
- I read everywhere that the effect of GLP-1RAs is glucose-dependent. That’s why they don’t cause hypoglycemia.
- Measuring your morning insulin in a fasted state (12h) would show whether GLP-1RAs increase insulin 24/7.
- You can also measure insulin C-peptide levels (half-life of C-peptide is ~30–35 min vs 3–5 min for insulin)
This Novo Nordisk paper in obese people is interesting: Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity 2017
If I understand correctly, semaglutide increases baseline insulin levels by ~40% (fasted state), but it decreases post-meal insulin levels by about 40% as well. Do we know if, for insulin, what matters is the time in range (not exceeding a certain threshold) or the area under the curve (“the lower the better”)? If it’s AUC, then semaglutide might be bad (+40% 24/7 but -40% 3 times per day after a meal). If it’s time in range and the +40% in fasted state is still below the “threshold” and the -40% post-meal helps people not going over the threshold, then it’s semaglutide is a net positive. (not sure if my reasoning is correct though…)
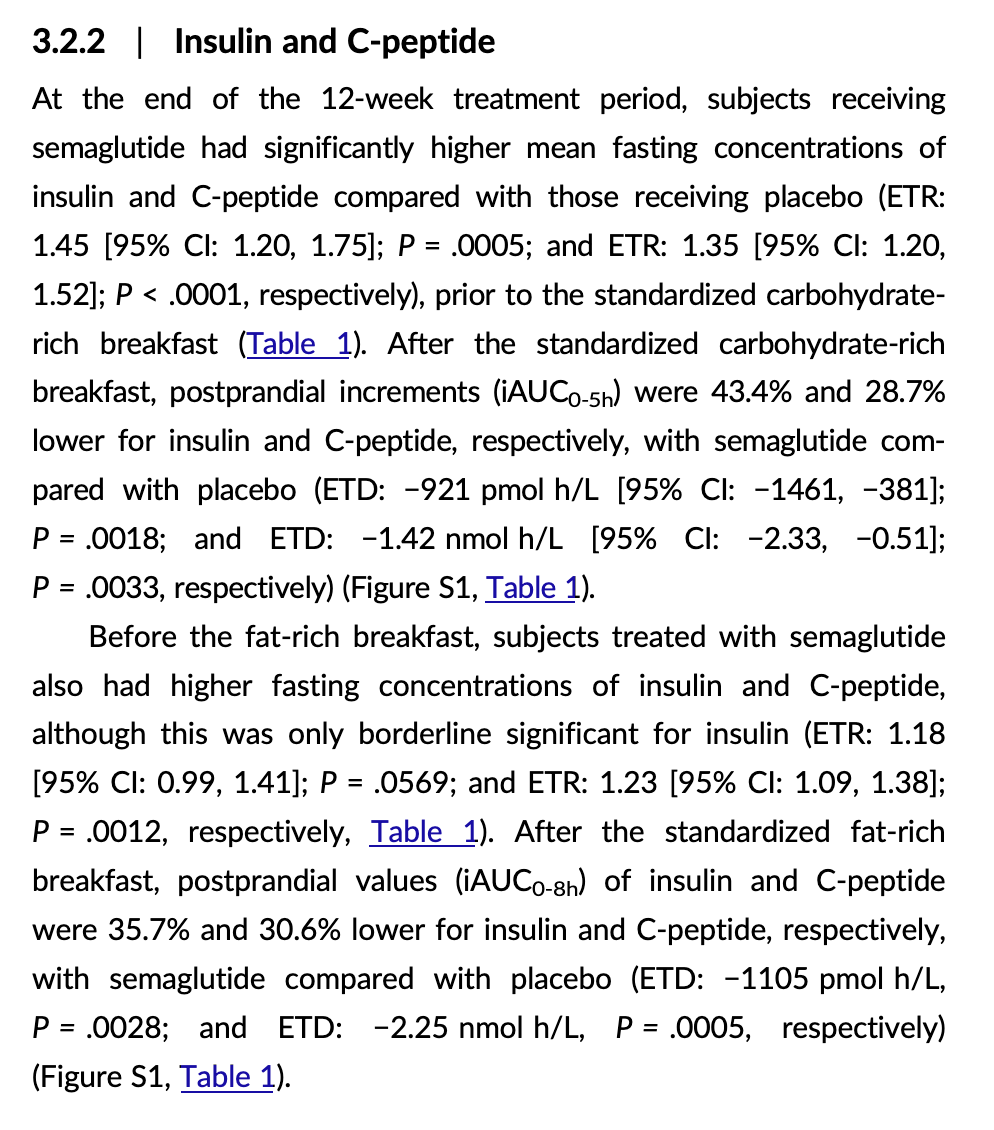
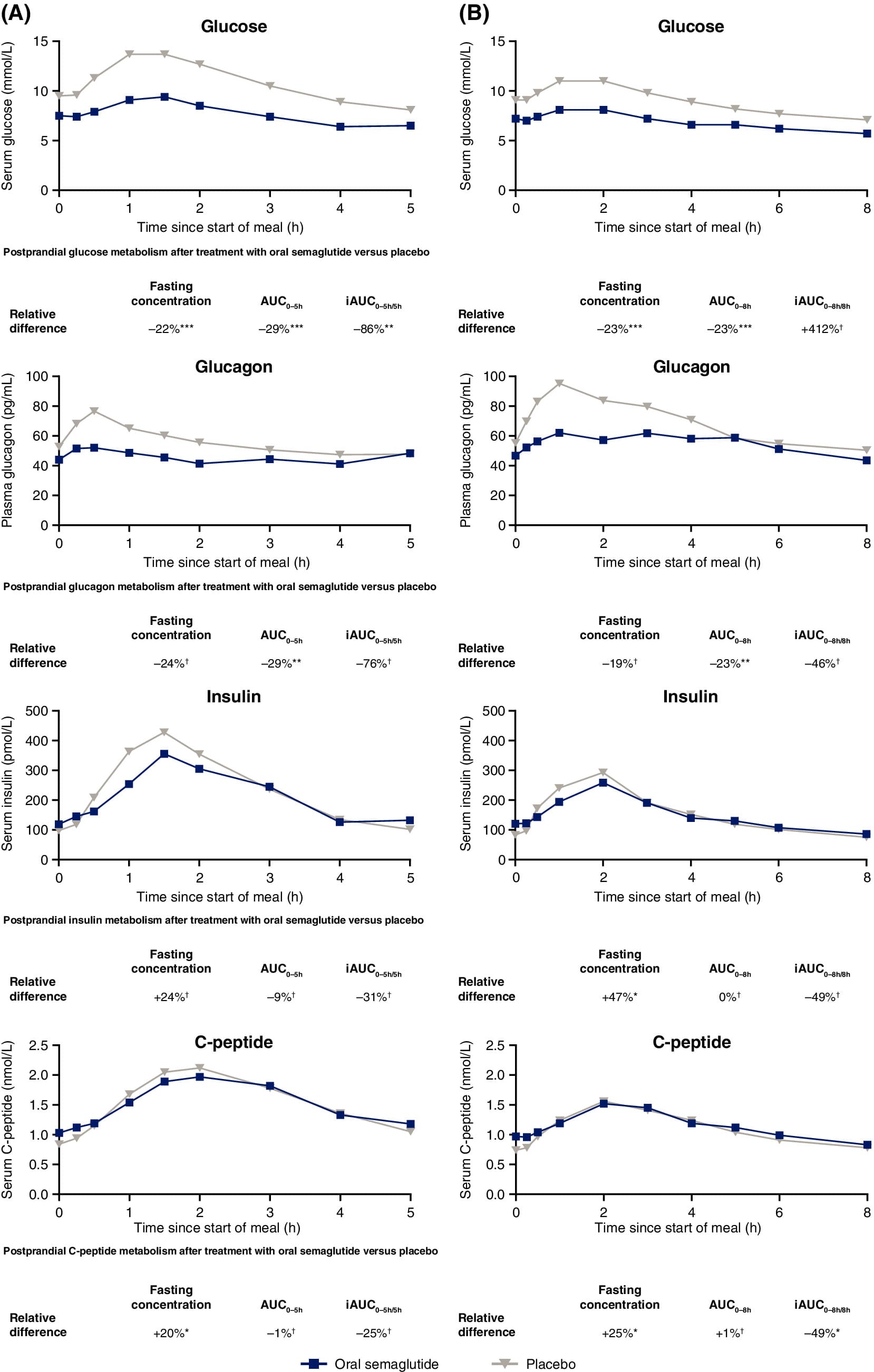
[EDIT, here’s a more recent Novo Nordisk paper on oral semaglutide in T2D: Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes 2021
It looks better for semaglutide here. I don’t get why the results BEFORE the standard breakfast and the fat-rich breakfast are different though.
I didn’t know that it could cause hypos. Do you have sources on this by any chance?
I noticed it when I was lying down on that arm in the sofa. And then replicated it many times. And think I also heard others here having noticed it. You can probably experiment with it yourself, just try lying on the arms/sensor at different angles for 5-7 minutes.
Here is the first thing that comes up when I googled it:
When pressure is applied to a CGM sensor, such as when a participant sleeps on their side and lays on the sensor, the CGM may read a false low value
Article - CGM is reading low values a....
Btw, what are your IGF-1 levels? If they are not as low as you’d like from a longevity phenotype you might be want to be a bit more concerned about risking to increase your insulin levels.
I think I’ve never measured it. What should I read about this?
perhaps start with some of these
IGF-1 inhibitors and lifespan extension?
Another (likely) Longevity Drug - Somavert / Pegvisomant
IGF-1 is Lower for Centenarians
A Life-Extension Drug for Big Dogs Is Getting Closer to Reality (Wired)
Great post and great questions. I don’t have good answers unfortunately.
Btw, one thing you might want to make sure you have considered is around known and unknown poly pharmacy risks/interactions of stacking increasing numbers of prescription drugs.
Some good news if you can get your doctor to prescribe it:
Costco will offer prescriptions for GLP-1 weight loss drugs to eligible members
The big-box retailer’s weight loss program is available “at the exclusive discount price of $179” for members
“The cost of medication is not included in the program’s base price. Without insurance, GLP-1s can cost between $950 and $1,600 per month, Sesame warned on its website.”
So that $179 is for the medical consultation only?
Ah - my bad. Thats definitely deceiving and clickbait … yes, only the consultation. I thought that was the pricing of the GLP1.
When Costco Members sign up for the weight loss program within the Sesame marketplace at the exclusive discount price of $179, they will:
- Receive three months of clinical consultation;
- select the clinician of their choice;
- have an initial live video consultation with the clinician;
- be able to message their clinician outside of scheduled appointments;
- receive a nutritional guide and recommendations;
- and be guided to an individualized, clinically-appropriate treatment program.
I forgot to answer this. As always, thanks a lot for your help!
Good paper published last week: Glucagon-like peptide 1 receptor agonist use and risk of thyroid cancer: Scandinavian cohort study 2024
The mean follow-up time was 3.9 years (standard deviation 3.5 years) in the GLP1 receptor agonist group and 5.4 years (standard deviation 3.5 years) in the DPP4 inhibitor group. 76 of 145 410 patients (incidence rate 1.33 events per 10 000 person years) treated with GLP1 receptor agonists and 184 of 291 667 patients (incidence rate 1.46 events per 10 000 person years) treated with DPP4 inhibitors developed thyroid cancer. GLP1 receptor agonist use was not associated with increased risk of thyroid cancer (hazard ratio 0.93, 95% confidence interval 0.66 to 1.31; rate difference −0.13, 95% confidence interval −0.61 to 0.36 events per 10 000 person years). The hazard ratio for medullary thyroid cancer was 1.19 (0.37 to 3.86). In the additional analysis comparing the GLP1 receptor agonist group with the SGLT2 inhibitor group, the hazard ratio for thyroid cancer was 1.16 (0.65 to 2.05).