More good news in the longevity field, this time from the Partridge lab at the Max Planck Institute for Biology of Ageing, where they combined two FDA-approved drugs with very good longevity results.
The researchers are, in this new pre-print paper, building on previous studies finding that lithium, trametinib and rapamycin can each extend lifespan in fruit flies (Drosophila), which is supported by other preliminary evidence in mice, worms, and cells, and observational findings in people.
The drugs all act on different cellular signalling pathways that together form the nutrient sensing network, which is conserved across evolution from worms and flies all the way to humans. This network adjusts what the body is doing in response to changes in nutrient levels. The drugs in question act on different proteins of this network to slow the ageing process and delay the onset of age-related death.
For the latest study, the researchers gave mice doses of trametinib and rapamycin, separately and in combination.
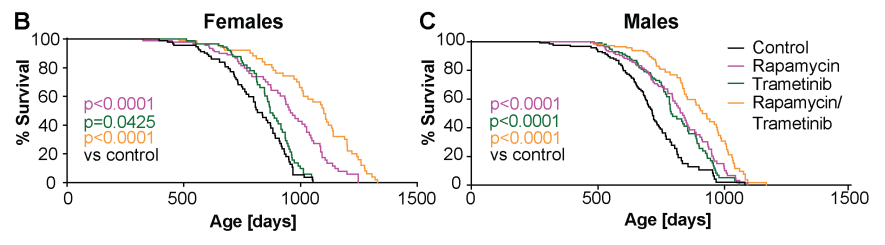
Trametinib treatment caused a significant lifespan extension in both sexes, in females, with a median lifespan extension of 7.2% but no significant effect on maximum lifespan, in males with an increase in median lifespan of 10.2% and maximum lifespan by 15.8%.
As previously shown, intermittent rapamycin treatment extended lifespan in both sexes with an increase in median and maximum lifespan of 17.4% and 16.5% respectively in females and 16.6% and 18.3% respectively in males.
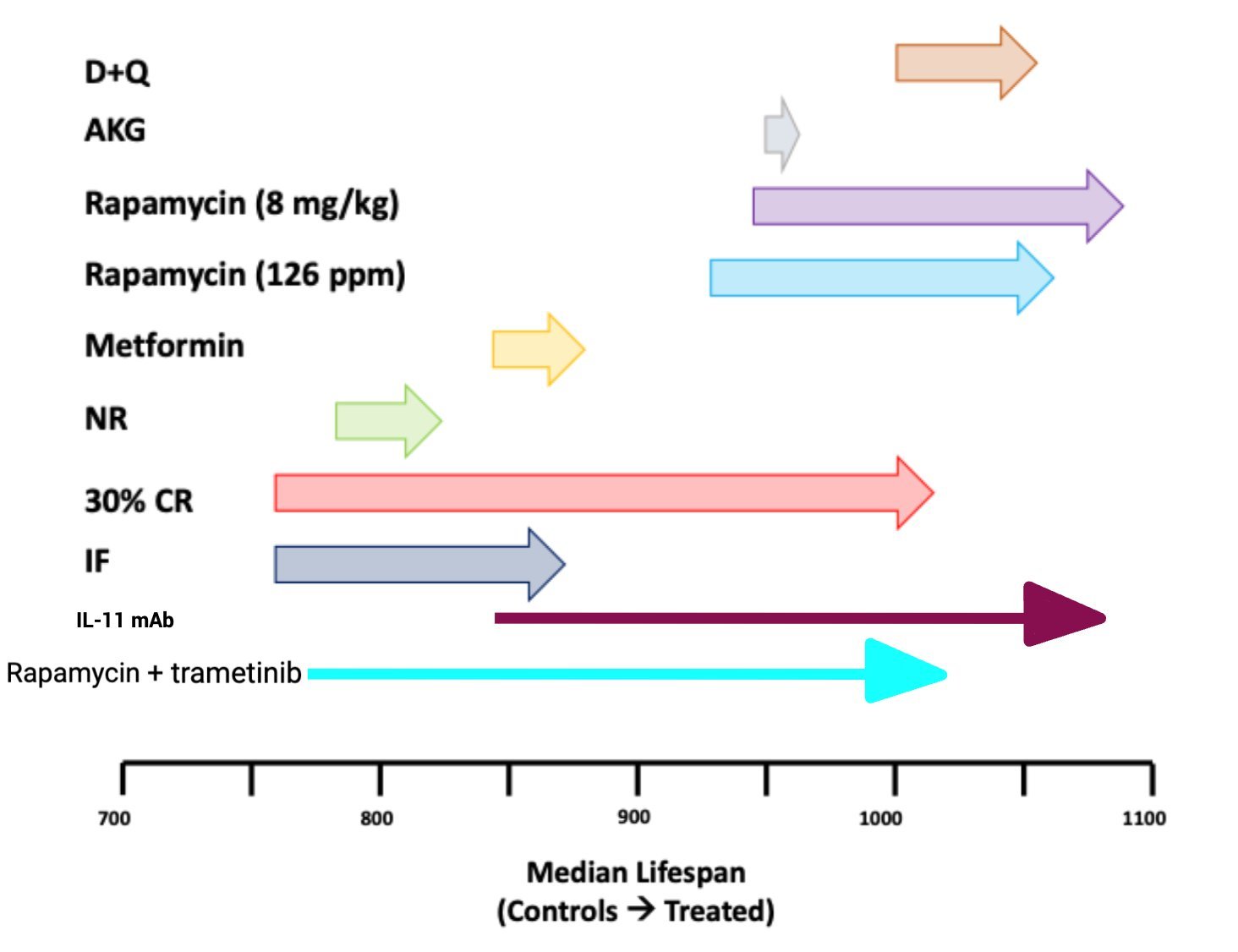
Combined treatment with rapamycin and trametinib increased survival more in females than in males. Combined treatment caused a larger increase compared to the single treatment in both sexes, with median and maximum lifespan increased by 34.9% and 32.4%, respectively, in females and by 27.4% and 26.1%, respectively, in males.
“combined trametinib and rapamycin treatment is more geroprotective than treatment with either drug alone, suggesting immediate translational potential for humans”
From the paper:
Combination treatment reduced liver tumours in both sexes and spleen tumours in males, and ameliorated the age-related increase in brain glucose uptake. There was a striking reduction in inflammation in the brain, kidney, spleen and muscle with combination treatment, accompanied by reduced circulating levels of pro-inflammatory cytokines. Trametinib alone is therefore geroprotective in mice, but combined trametinib and rapamycin treatment is more geroprotective than treatment with either drug alone, suggesting immediate translational potential for humans.
Full Pre-print Paper:
https://www.biorxiv.org/content/10.1101/2024.07.25.605097v1